A clinical trial involves complex overlapping processes, many of which require specialized expertise and experience. So how can you run a clinical trial successfully if you don’t have the expertise in-house? That’s where a CRO comes in.
What Is a CRO?
A contract research organization, or CRO, supports the biotech, medical devices and pharma industries by providing clinical research services. Working with a CRO can be a cost-effective way of ensuring the expertise and experience needed, especially for companies carrying out research in niche markets.

The services a CRO provides vary depending on what the client needs. They can cover the span of the clinical research process, from preclinical research to pharmacovigilance (clinical trial phases I to IV) and other services, such as commercialization and the development of assays and biopharmaceuticals.
Since CROs range in size, from small organizations to large multinationals. They often specialize in certain areas – especially the smaller organizations. For example, they may provide services in specific areas, or focus on a number of therapeutic areas. By specializing like this, CROs can ensure they have the expertise needed in the areas in which they work.
The CRO’s Role In The Clinical Trial Process
One specific area in which CROs support clients is setting up, managing and closing clinical trials. CROs that specialize in clinical trials – including Siron Clinical – often work on every part of the process, from setup, which requires expertise in regulations, to FDA or EMA approval and ongoing monitoring.
According to the definition of the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), a CRO is: “A person or an organization (commercial, academic, or other) contracted by the sponsor to perform one or more of a sponsor’s trial-related duties and functions.”
The sponsor has a central role throughout the clinical trial process. Although regulatory tasks differ in the EU and the US, they include initiating the trial process by submitting an application and ensuring approval is obtained (directly or indirectly). The sponsor is responsible for managing and financing the trial.
The ICH Guideline for Good Clinical Practice (GCP) specifies the responsibilities a CRO can take over from a sponsor:
- 5.2.1: A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a CRO, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. The CRO should implement quality assurance and quality control.
- 5.2.2: Any trial-related duty and function that is transferred to and assumed by a CRO should be specified in writing. The sponsor should ensure oversight of any trial-related duties and functions carried out on its behalf, including trial-related duties and functions that are subcontracted to another party by the sponsor’s contracted CRO(s).
- 5.2.3: Any trial-related duties and functions not specifically transferred to and assumed by a CRO are retained by the sponsor.
- 5.2.4: All references to a sponsor in this guideline also apply to a CRO to the extent that a CRO has assumed the trial-related duties and functions of a sponsor.
How Siron Clinical Can Support You
As a CRO specialized in clinical trials, Siron Clinical can support your trial with services covering the entire process.
- Project Management, including developing and tracking study timelines, ensuring all activities are within scope and budget, coordinating and executing Clinical Trial activities and acting as the central point of contact for the client.
- Clinical Monitoring, including protocol, regulatory and ICH GCP compliance verification, site personnel training and pharmacovigilance: assisting and ensuring appropriate reporting of CIOMS, DSURs, annual IDB updates.
- Quality Assurance / Control, including clinical study document review, accompanied site visits and inspection preparation site visits.
- Regulatory Submissions, including regulatory advice, preparation and submission of dossiers, and acting as EU Legal or Data Controller representative.
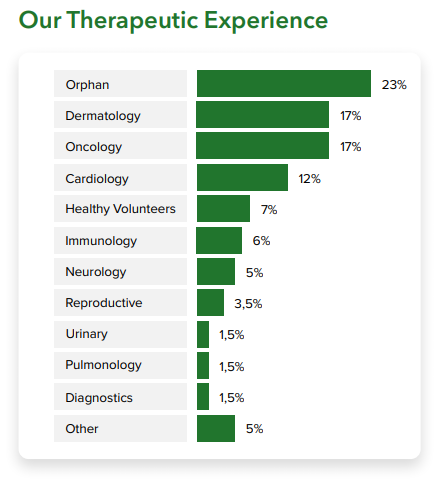
You can benefit from these services across a range of therapeutic areas in which we have experience. Over the last 20 years, we have worked on 120 complex clinical trials, including orphan indications, pediatric, cardiovascular, oncology, hepatology and dermatology studies.
To find out how Siron Clinical can support your clinical trial, contact our team.




0 Comments