We recently published a free eBook on EU clinical trial regulations. We share some of our insights from the eBook in the infographic below. Learn about the differences between the US and EU regulatory submission process, and discover how to get your plan in order.
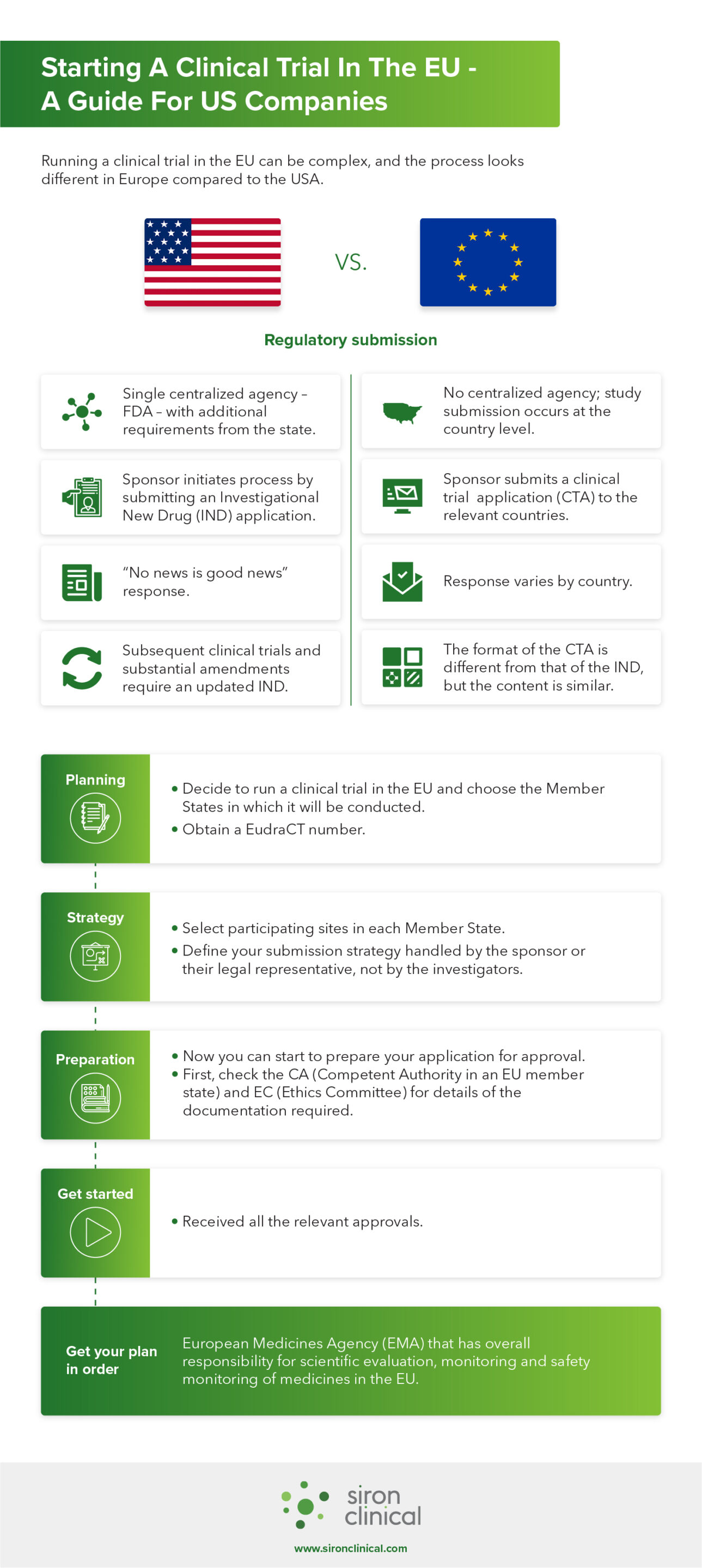
Did we spark your interest? Download the full eBook and learn more about the EU regulations concerning clinical trials. You will read in more detail about insights from our experience and have access to a handy list with tools and resources when starting clinical trial in the EU.





0 Comments